How proteins know where to go
Chaperones. Transporters. Receptors. Proteins sound like they know what they’re doing. But proteins rarely function in isolation: virtually all protein functions involve multiple proteins interacting in complexes. For example, DNA replication requires helicase to unwind the DNA double helix, topoisomerase to relieve torsional stress, a sliding clamp and clamp loader, and dozens other kinds of proteins packaged in a complex called a replisome. The nuclear pore complex, which controls traffic between the nucleus and the cytoplasm, has about a thousand proteins in humans. How do proteins know how to find their complementary molecules in a cell?
It turns out that they don’t. Proteins just diffuse through a cell, getting kicked around by other molecules. The first thing to note is that though this slows things down — a protein naturally moves at 5 m/s, fast enough to travel its own length in about a nanosecond, but its random walk means it takes almost a thousand times longer to get anywhere — motion is still very fast compared to our familiar world. David Goodsell gives this example in The Machinery of Life:
[I]magine a typical bacterial cell […] and place an enzyme at one end and a sugar molecule at the other. They will bump around and wander through the whole cell, encountering many molecules along the way. On average, though, it will only take about a second for those two molecules to bump into each other at least once. This is truly remarkable: this means that any molecule in a typical bacterial cell, during its chaotic journey through the cell, will encounter almost every other molecule in a matter of seconds.
The far bigger problem is: if proteins are just randomly diffusing, instead of getting transported in dedicated channels, how do they ever find their targets? The answer is complementarity. The vast majority of molecule pairings will have the two just weakly interact. A protein will form the right hydrogen bonds and salt bridges, and contort into the right hydrophobicity-minimizing shape, with only a specific handful other proteins.
Here’s Goodsell’s illustration of chemical complementarity in enolase. The two subunits interlock:
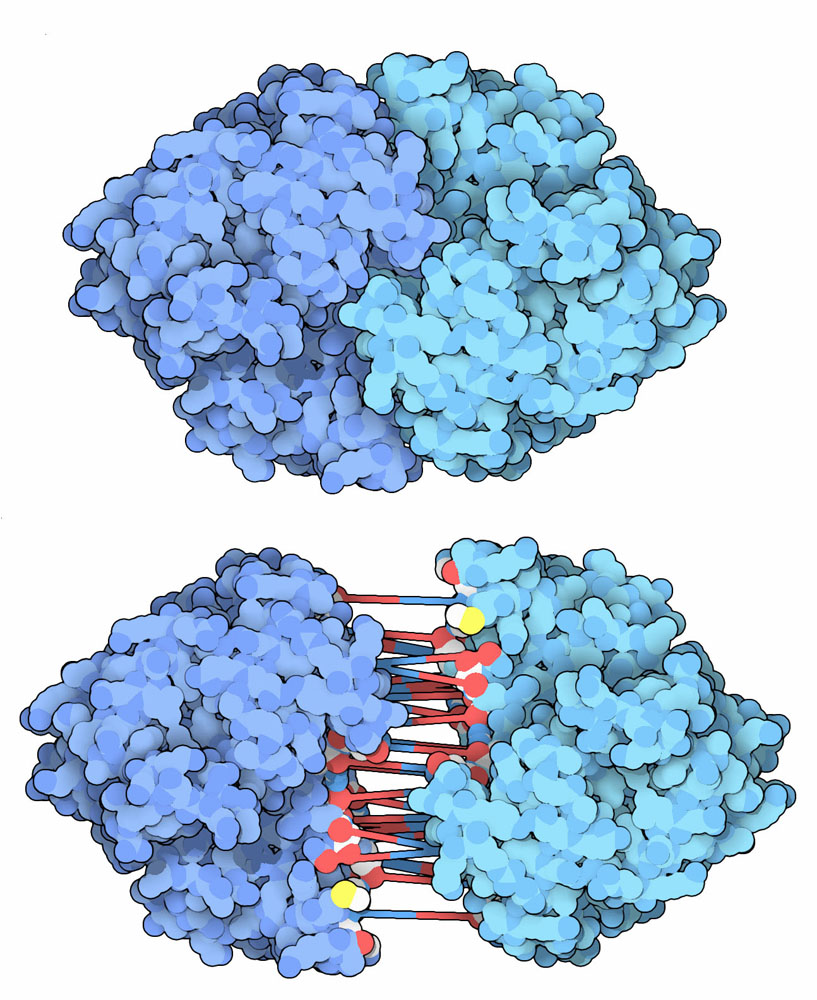
In addition, there’s very clever design to speed along proteins finding their complements: dimensionality reduction.
If a protein binds weakly to a membrane, it can bind at one spot and then hop around on the membrane surface. Since this protein only needs to move in two dimensions on the surface, the molecule diffuses over a smaller region of space than if it were free to diffuse in three dimensions. So, it can find its targets on the membrane surface very quickly. This is also the case for proteins that bind to DNA — they can move rapidly up and down the helix finding the proper place. For instance, the lac repressor protein shows a weak, non-specific binding for most sections of DNA, and a very strong binding for the particular sequence of nucleotides it is designed to repress. Inside cells, by sliding up and down the helix, it finds its preferred binding site hundreds of times faster than expected with random, three-dimensional diffusion.
This is how, as Goodsell puts it, “the whole thing can […] be orchestrated by random association in the teeming cytoplasmic soup.”